
An HIV urine self-test kit called the Human Immunodeficiency Virus (HIV) type-I urine antibody diagnostic kit (colloidal gold), co-developed by Xiamen University and Beijing Wantai Biological Pharmacy Enterprise, recently obtained the prequalification of medical products from the World Health Organization, known as WHO PQ.
It is the world's first HIV urine self-test kit to obtain WHO PQ and is also the third HIV testing product jointly developed by both parties to obtain the certification.
There are currently over one million new cases of HIV infection worldwide every year and millions of HIV infected individuals remain undetected and undiagnosed.
WHO believes that high-quality testing services remain a key pathway for detecting, treating and preventing HIV. It further emphasized the strengthening of self-testing in its 2024 updated HIV testing guidelines.
In 2009, XMU and the Xia Ningshao team from the Xiang An Biomedicine Laboratory completed the technical validation of HIV urine testing reagents. This was to address the sticking points of inconvenient operation and insufficient sensitivity of existing dry blood spots and saliva self-testing reagents.
After a decade of clinical validation using a large sample system, the HIV urine self-testing reagent was approved for sale in 2019, becoming the world's first HIV urine testing reagent available for the public to self-test.
Moreover, the kit has significant advantages of non-invasiveness, strong confidentiality and easy operation.
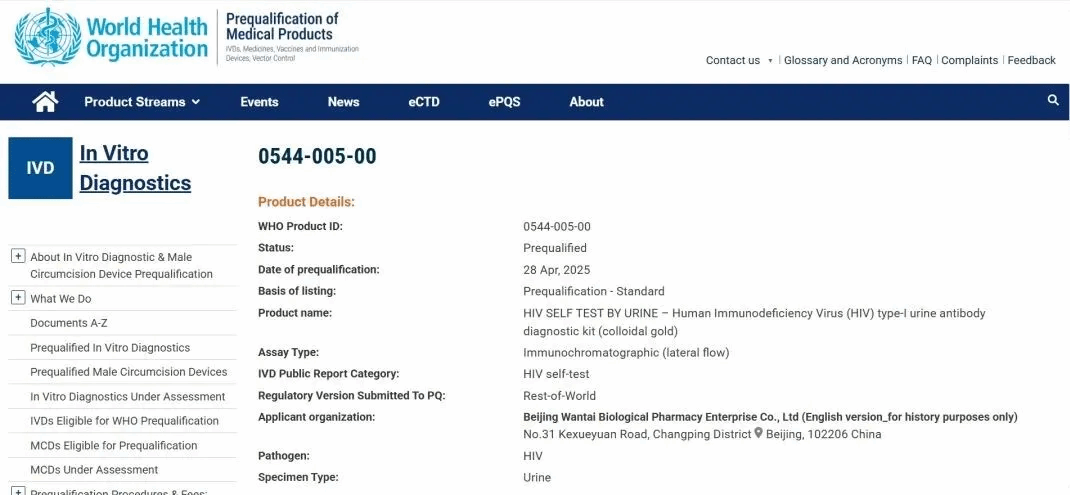
The HIV urine self-test kit, co-developed by Xiamen University and Beijing Wantai Biological Pharmacy Enterprise, obtains WHO PQ. [Photo/WeChat account: xmu_1921]