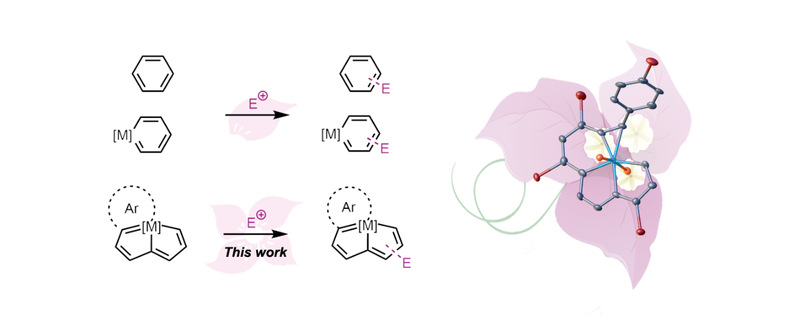
Electrophilic Aromatic Substitution Reactions of Compounds with Craig-Möbius Aromaticity
Hong Zhang, Haiping Xia and colleagues have successfully demonstrated Electrophilic Aromatic Substitution (EAS) reactions of Craig-Mobius aromaticity, osmapentalenes and fused osmapentalenes. The highly reactive nature of osmapentalene makes it susceptible to electrophilic attack by halogens, and thus osmapentalenes, osmafuran-fused osmapentalene and osmabenzene-fused osmapentalene can undergo typical EAS reactions.
EAS reactions are characteristic reactions of aromatic species and useful in synthetic chemistry. The EAS reactions of complexes with Hückel aromaticity are well established, and however no comparable reaction has been developed for molecules with Craig-Mobius aromaticity.
The selective formation of a series of halogen substituted metalla-aromatics via EAS reactions has revealed an unprecedented approach to otherwise elusive compounds such as the cyclic chlorirenium ions. In addition, density functional theory calculations were conducted to study the electronic effect on the regioselectivity of the EAS reactions. (Proc. Natl. Acad. Sci. U. S. A., 2021, 118, e2102310118)